The drug is the first and only monotherapy for adults with major depressive disorder
Johnson & Johnson has announced that the U.S. Food and Drug Administration has approved Spravato (esketamine) CIII nasal spray as the first and only monotherapy for adults with major depressive disorder who have not responded adequately to at least two oral antidepressants.
The company hailed the approval as a significant advancement in the treatment of MDD, a condition affecting approximately 21 million adults in the United States.
Major depressive disorder is one of the most prevalent psychiatric disorders, and about one-third of patients do not respond to traditional oral antidepressants, severely impacting the quality of life and contributing to the high economic burden associated with treatment-resistant depression.
Image below via Johnson & Johnson
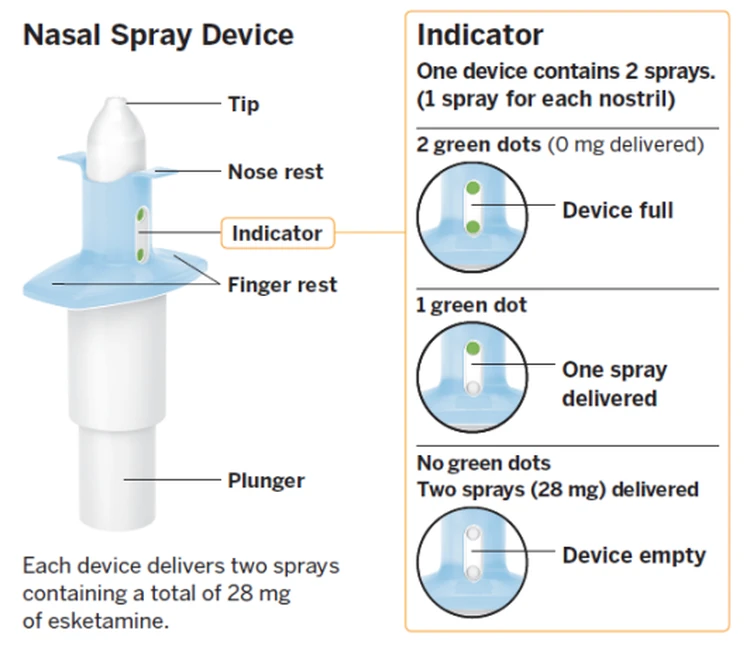
Complicated treatment
"Treatment-resistant depression can be very complicated, especially for patients who do not respond to oral antidepressants or cannot tolerate them, said Bill Martin, head of Neuroscience at Johnson & Johnson Innovative Medicine.
Spravato is now available as a standalone treatment, meaning patients may experience improvements in depressive symptoms as early as 24 hours and at 28 days without the need for daily oral antidepressants."
The FDA's decision followed a priority review and was based on positive outcomes from a randomized, double-blind, placebo-controlled study.
Johnson & Johnson said the study demonstrated that Spravato significantly improved depressive symptoms compared to placebo, with 22.5% of patients achieving remission at week four, compared to 7.6% in the placebo group. The safety profile of SPRAVATO was consistent with previous data, with no new safety concerns identified.
The new drug is available only through a restricted program known as the Spravato Risk Evaluation and Mitigation Strategy Program.
Photo Credit: Consumer Affairs News Department Images
Posted: 2025-01-21 15:15:40




















